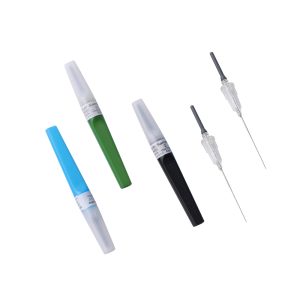
Blood Collection Tube
- Human venous blood samples collection container for single use consists of tube,piston,tube cap, and additives;for products containing additives,additives should conform to the requirements of relevant laws and regulations.A certain amount of negative pressure is maintained in the blood collection tubes;therefore,while using with the disposable venous blood collection needles,it can be used to collect the venous blood by the principle of negative pressure.
- 2ml~10ml,13×75mm,13×100mm,16×100mm,coagulation-promotion tube and anticoagulation tube.
- Total closed system,avoiding cross infection,provide a safety working environment.
- In accordance to international standard,washing by deionized water and sterilized by Co60.
- Standard color,easy identification for difference use.
- Safety designed,preventing spattering of blood.
- Pre-set vacuum tube,automatic performance,easily operation.
- Unified size,more convenience to use.
- Internal wall of tube are special treated,thus the tube be smoother,low effect on blood cell integration and configuration,no fibrinad sorption,no hemolysis quality specimen in adopted.
Product Details
Blood Collection Tube Model
| VT-01 |
Specification
| Color Codes | Collecting Blood Volume(ml) | Additive | Clinic Use |
|---|---|---|---|
| Red | 2.0, 3.0, 4.0, 5.0 |
Non(Inner Wall With Silicon keton) | Serum Biochemical Blood Storeroom Experiment |
| Orange | 3.0, 4.0, 5.0 |
Coagulant | Celerity Serum Biochemical |
| Gold | 3.0, 4.0, 4.5, 5.0 |
Gel Clot Activator |
Celerity Serum Seperate Biochemical,Medication Dyn Experiment |
| Green | 2.0, 3.0, 4.0, 5.0 |
Heparin | Celerity Plasm Biochemical, Blood Stream Change Experiment |
| Lavender | 1.0, 2.0, 2.5, 5.0 |
EDTA K2 Spray or Liquid |
Haemetolog,Whole Blood Experiment,Blood Stream Change Experiment |
| Lt Blue | 1.8, 2.7 | Coagulant | Blood Solidification Experiment, PT, PTT, Cruor Gene Experiment |
| Black | 1.6, 2.4 | E.S.R. | Handworked Blood Sink Experiment,Automatic Blood Sink Experiment |
| Grey | 3.0, 4.0, 5.0 | Glucose | Blood Sugar Experiment |
Packing
| 1800 pcs/carton |
Product Features
| Intended Use | As a venous blood collection system,a disposable human venous blood collection container is used with blood collection needle and needle holder for the collection, storage,transport and pretreatment of blood samples for venous serum,plasma or whole blood testing in clinical laboratory. |
| Structure and Composition | Human venous blood samples collection container for single use consists of tube,piston,tube cap,and additives;for products containing additives. |
| Main Material | The test tube material is PET material or glass,the rubber stopper material is butyl rubberand the cap material is PP material. |
| Shelf Life | The expiry date is 12 months for PET tubes; The expiry date is 24 months for glass tubes. |
| Certification and Quality Assurance | Quality System Certificate: ISO13485(Q5 075321 0010 Rev. 01) TÜV SÜD The IVDR has submitted the application,pending review. |