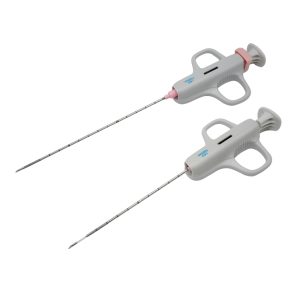
Nerve Block Needle (Ultrasound-Guided)
- This nerve block needle syringe is made of SUS304 stainless steel.
- The syringe has a thin wall,a large internal diameter,and a high flow rate.
- The conical connector is desig to the 6:100 standard, ensuring good compatibility with medical devices.
- Precise positioning.
- Reduced puncture difficulty.
- Short onset time.
- Visual operation with accurate dosage control.
- Reduced systemic toxicity and nerve damage.
Product Details
Product Features
| Intended Use | This product provides safe and precise ultrasound-guided needle placement for drug delivery. |
| Structure and Compostion | The product is composed of a protective sheath,a graduated syringe,a needle hub,conical adapters,tubing,a conical interface,and an optional protective cap. |
| Main Material | PP,PC,PVC,SUS304. |
| Shelf Life | 5 years |
| Certification and Quality Assurance | In compliance with Medical Devices Directive 93/42/EEC(Class IIa). Manufacturing process is in compliance with ISO 13485 and ISO9001 Quality System. |
Specification
| Metric (mm) | Imperial |
| 0.7 | 22G |
| 0.8 | 21G |