Insulin Pen Needles
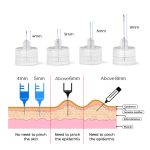
- 29-33G,needle length 4mm-12mm,thin wall/regular wall.
- Double point system.
- Sterile,non-toxic,non-pyrogenic.
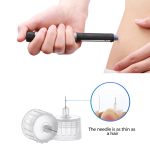
- Safety design and easy for using.
- Better penetration make the injection more comfortable.
- High compatibility and cover almost all branches.
Product Details
Model
| IPN01 |
Specification
| OD | Gauge | Color Code | General Specifications | Packing Quantity |
|---|---|---|---|---|
| 0.3 | 30G | Yellow | 0.30(0.30×6mm/30G) | 10000 pieces / box |
| 0.25 | 31G | Blue | 0.25(0.25×5mm/31G) | 10000 pieces / box |
| 0.23 | 32G | Green | 0.23(0.23×4mm/32G) | 10000 pieces / box |
| 0.2 | 33G | Purple | 0.20(0.20×4mm/33G) | 10000 pieces / box |
Packing
| 100pcs/box | 10000pcs/carton | Carton Size:505×385×370mm | G.W 13kg |
Product Features
| Intended Use | Insulin pen needle is for use with pre-diabetic insulin liquid filed insulin pen for insulin injection. |
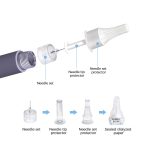
| Structure and Composition | Needle set,needle tip protector,needle set protector,sealed dialyzed paper. |
| Main Material | PE,PP,SUS304 Stainless Steel Cannula,Silicone Oil |
| Shelf Life | 5 years |
| Certification and Quality Assurance | Conform to ISO11608-2. In compliance with European Medical Device Directive 93/42/EEC(CE Class: Ila). Manufacturing process is in compliance with ISO 13485 and ISO9001 Quality System. |