Hypodermic & Connector Engineering Excellence
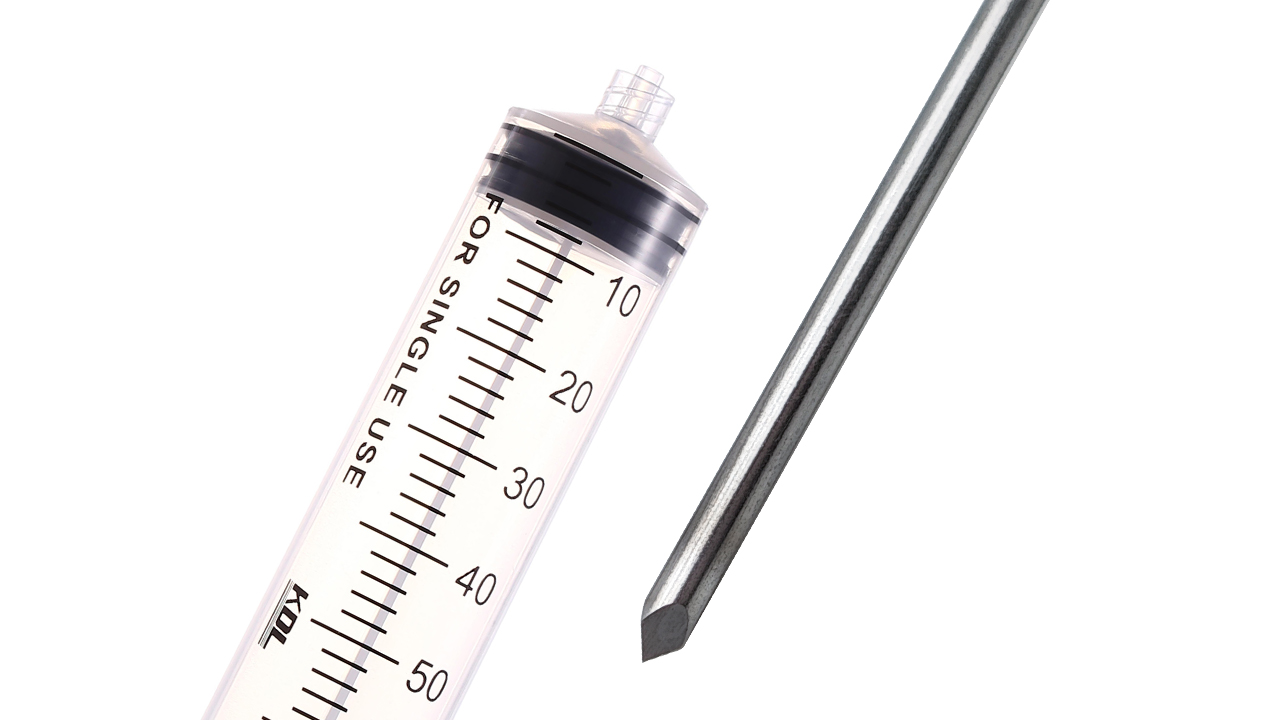
Medical Needles & Syringe
Ultra-Sharp Penetration: 30G to 18G hypodermic needles with electropolished surfaces (Ra ≤ 0.2µm).
Customization: Bevel angles (12°–30°), lengths (5mm–150mm), and hub designs (Luer Lock/Slip, Catheter).
Compliance: ISO 7864 (sterile) & ISO 9626 (stainless steel) validated.

Connectors & Tubing Systems
Leak-Proof Performance: Luer taper connectors tested per ISO 594-1/594-2.
Material Versatility: USP Class VI silicone, PVC-free thermoplastic elastomers (TPE), or biocompatible PU.
Custom Kitting: Pre-assembled IV sets, catheter extension lines with RFID tracking
Solution
Our Service

Understanding Your Needs
Precision Starts with Your Requirements
Regulatory-First Approach: Dedicated team navigates country-specific compliance (e.g., FDA 510(k), CE Marking, MDR).
Needs Analysis Workshop: Joint sessions to align technical specs,budget, and certification timelines.
Free Regulatory Consultation: Pre-audit support for your local market entry requirements.
IP Protection Guarantee: NDAs and secure data protocols(GDPR compliant).

Prototyping & Sampling
From CAD to Certified Samples in 72 Hours
Rapid Prototyping: CNC machining/3D printing for syringe components (tolerance ±0.01mm).
Pre-Shipment Testing: Samples validated against ISO 7864/ISO 9626 standards.
Free Global Courier: DHL/FedEx prepaid return service for sample approvals.
DFM Optimization: Reduce production costs by 15% through design enhancements.

Production & Inventory
Smart Manufacturing, Assured Quality
Zero-Defect Pledge: 7-stage QA process including vision inspection and leak testing.
Flexible MOQs: Start from 5,000 units with 30% safety stock for urgent orders.
Real-Time Monitoring: IoT-enabled production tracking via client portal.
Green Manufacturing: Energy-saving injection molding systems (30% lower carbon footprint).

Shipping & Logistics
Door-to-Door Medical Logistics Mastery
Global Network: Serve 50+ countries with DDP (Delivered Duty Paid) terms.
Cold Chain Solutions: Temperature-controlled shipping for sensitive devices.
Documentation Hub: Automated export docs (COO, SDS, commercial invoice).
98.7% On-Time Delivery: Real-time GPS tracking + customs pre-clearance.

After-Sales Support
Lifelong Partnership, Beyond Delivery
24/7 Multilingual Support: Engineers fluent in EN/ES/FR/AR.
Extended Warranty: 3-year product liability coverage.
RCA (Root Cause Analysis): Issue resolution within 48 hours.
Risk-Mitigated Procurement Partnerships
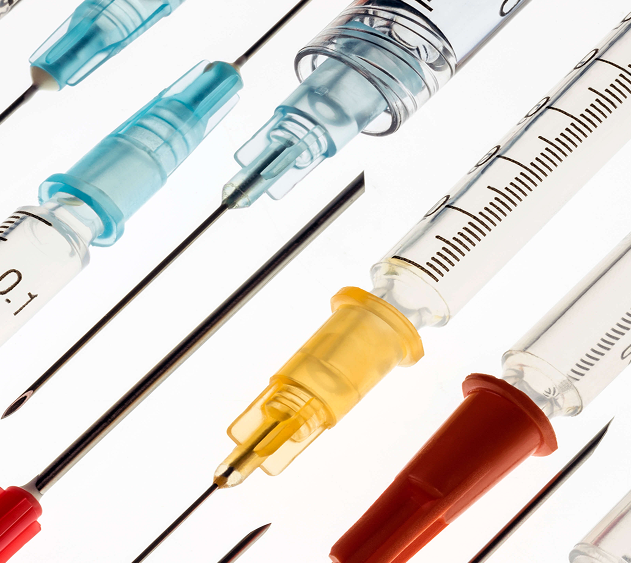

Vertical Integration: Raw material welding → Precision wall reduction → Annealing in our FDA-registered facility (Process 01–03).

Zero-Defect Guarantee: 7-stage QA including particle testing (USP <788>) and tensile strength validation.

260+ Active Patents | 106+ Product Registrations (CE MDR,FDA 510(k), ANVISA).

Dual Certifications: ISO 13485 (Medical Devices) + ISO 15378 (Primary Packaging).

Recyclable Packaging: PCR plastics for trays/clamshells (ISO 14001 compliant).

Energy-Efficient Molding: 25% lower energy consumption via servo-electric systems.
Becoming a global leader in medical puncture devices
We are committed to pursuing technological innovation and continuous improvement to build the KDL brand, which is recognized and trusted by users around the world.
We believe that through our extraordinary efforts and insights, our products can make the lives of millions of users healthier and better through better treatment and care.
This is the vision of KDL and the common pursuit of each and every one of us.
ISO
End-to-End Manufacturing Expertise:
Full industrial chain from raw material welding to precision needle processing (ISO 13485 & FDA-compliant).
260+
Certified Excellence:
260+ global patents, CMDC/EU TUV/FDA certifications, and 106+ product registrations worldwide.
30+
Scalable Capacity:
30+ years of OEM/ODM experience with fully automated production lines.
35%
Cost Efficiency:
Optimized supply chain reduces lead times by 35% vs industry benchmarks.
100%
Sustainability Commitment:
ESG-aligned processes with 100% recyclable packaging.
 +86-791-8686-1216
+86-791-8686-1216 




